How Do Skin Cells Divide
To back up its specialized functions, the skin has basic requirements that must exist satisfied for almost every tissue. It needs mechanical force, largely provided by a supporting framework of extracellular matrix, mainly secreted by fibroblasts. It needs a blood supply to bring nutrients and oxygen and remove waste product products and carbon dioxide, and this requires a network of blood vessels, lined with endothelial cells. These vessels also provide access routes for cells of the immune organization to provide defenses against infection: macrophages and dendritic cells phagocytose invading pathogens and assistance actuate lymphocytes, which mediate more than sophisticated adaptive immune system responses (discussed in Affiliate 24). Nerve fibers are needed too, to convey sensory information from the tissue to the central nervous organization, and to evangelize signals in the contrary management for glandular secretion and smooth musculus contraction.
Figure 22-1 illustrates the compages of the tissue and shows how it makes provision for all these support services. Peel consists of 2 chief parts: an epithelium, the epidermis, lying outermost, and beneath this a layer of connective tissue, which includes the tough collagen-rich dermis (from which leather is made) and the underlying fat subcutaneous layer or hypodermis. In the pare, as elsewhere, the connective tissue, with vessels and nerves running through it, is responsible for nigh of the general supportive functions listed above.

Figure 22-ane
Mammalian skin. (A) These diagrams show the cellular compages of thick skin. (B) Micrograph of a cantankerous department through the sole of a human pes, stained with hematoxylin and eosin. The skin can be viewed as a large organ composed of two main tissues: (more than...)
The defining component of the skin—the specialized tissue that is peculiar to this organ, even though not the major office of its majority—is the epidermis. This has a simple organization, and it provides a cute introduction to the way in which tissues of the adult body are continually renewed, through processes like to those that operate in the embryo. We render to connective tissues after.
Epidermal Cells Form a Multilayered Waterproof Bulwark
The epidermis suffers more than direct, frequent, and damaging encounters with the external world than any other tissue in the body. Its need for repair and renewal is central to its organization.
The epidermis is a multilayered (stratified) epithelium composed largely of keratinocytes (so named considering their characteristic differentiated activity is the synthesis of intermediate filament proteins chosen keratins, which give the epidermis its toughness) (Figure 22-2). These cells modify their appearance from one layer to the next. Those in the innermost layer, attached to an underlying basal lamina, are termed basal cells, and it is usually merely these that divide. Above the basal cells are several layers of larger prickle cells (Effigy 22-iii), whose numerous desmosomes—each a site of anchorage for thick tufts of keratin filaments—are just visible in the light microscope every bit tiny prickles effectually the jail cell surface (hence the name). Beyond the prickle cells lies the thin, darkly staining granular cell layer (encounter Figure 22-two). Information technology is at this level that the cells are sealed together to form a waterproof barrier, fulfilling the about fundamentally important of all the functions of the epidermis. Mice that fail to grade this bulwark because of a genetic defect dice from rapid fluid loss soon after nascence, even though their skin appears normal in other respects.

Figure 22-2
The multilayered construction of the epidermis, equally seen in a mouse. The outlines of the keratinized squames are revealed by swelling them in a solution containing sodium hydroxide. The highly ordered hexagonal arrangement of interlocking columns of cells (more...)

Figure 22-3
A prickle cell. This drawing, from an electron micrograph of a section of the epidermis, shows the bundles of keratin filaments that traverse the cytoplasm and are inserted at the desmosome junctions that bind the prickle cell (blood-red) to its neighbors. (more...)
The granular layer, with its bulwark to the move of water and solutes, marks the boundary between the inner, metabolically agile strata and the outermost layer of the epidermis, consisting of dead cells whose intracellular organelles take disappeared. These outermost cells are reduced to flattened scales, or squames, filled with densely packed keratin. The plasma membranes of both the squames and the outer granular cells are reinforced on their cytoplasmic surface past a thin (12 nm), tough, cantankerous-linked layer of proteins, including a cytoplasmic protein called involucrin. The squames themselves are unremarkably so compressed and sparse that their boundaries are difficult to make out in the light microscope, but soaking in sodium hydroxide solution (or a warm bathroom tub) makes them smashing slightly, and their outlines can then be seen (see Figure 22-2).
Differentiating Epidermal Cells Synthesize a Sequence of Different Keratins as They Mature
Having described the static moving-picture show, allow us at present set information technology in motion and see how the epidermis is continually renewed by the production of new cells in the basal layer. While some basal cells are dividing, adding to the population in the basal layer, others (their sisters or cousins) are slipping out of the basal cell layer into the prickle cell layer, taking the starting time step on their outward journey. When they reach the granular layer, the cells first to lose their nucleus and cytoplasmic organelles, through a degradative mechanism that involves partial activation of the machinery of apoptosis; in this mode, the cells are transformed into the keratinized squames of the keratinized layer. These finally flake off from the surface of the peel (and go a main elective of household dust). The menstruum from the time a jail cell is built-in in the basal layer of the homo peel to the time it is shed from the surface is of the order of a month, depending on the region of the torso.
The accompanying molecular transformations can be studied past analyzing either thin slices of epidermis cutting parallel to the surface or successive layers of cells stripped off by repeatedly applying and removing strips of agglutinative tape. The keratin molecules, for example, which are plentiful in all layers of the epidermis, can be extracted and characterized. They are of many types (discussed in Affiliate 16), encoded past a large family of homologous genes, with the multifariousness further increased through alternative RNA splicing. As the new keratinocyte in the basal layer is transformed into the squame in the outermost layers (see Figure 22-3), it switches from one choice of keratins to another. Meanwhile other feature proteins, such equally involucrin, besides begin to be synthesized as part of a coordinated program of terminal cell differentiation—the process in which a precursor cell acquires its final specialized characteristics and unremarkably permanently stops dividing. The whole program is initiated in the basal layer. Information technology is here that the fates of the cells are decided.
Epidermis Is Renewed by Stem Cells Lying in Its Basal Layer
The outer layers of the epidermis are replaced a thousand times over in the course of a human lifetime. In the basal layer at that place accept to be cells that tin can remain undifferentiated and conduct on dividing for this whole flow, continually throwing off descendants that differentiate, leave the basal layer, and are eventually discarded. The process can be maintained only if the basal cell population is self-renewing. Information technology must therefore contain some cells that generate a mixture of progeny, including daughters that remain undifferentiated like their parent, as well as daughters that differentiate. Cells with this property are called stem cells. They have so important a part in such a variety of tissues that it is useful to have a formal definition.
The defining properties of a stem prison cell are equally follows:
- 1.
-
Information technology is non itself terminally differentiated (that is, it is not at the end of a pathway of differentiation).
- 2.
-
It tin can divide without limit (or at least for the lifetime of the animate being).
- three.
-
When it divides, each daughter has a choice: it can either remain a stalk jail cell, or information technology can commence on a course that commits it to concluding differentiation (Figure 22-iv).

Figure 22-four
The definition of a stem prison cell. Each daughter produced when a stalk cell divides can either remain a stem prison cell or keep to become terminally differentiated. In many cases, the daughter that opts for last differentiation undergoes additional cell divisions (more than...)
Although it is function of the definition of a stem cell that it should be able to divide, it is not part of the definition that information technology should dissever rapidly; in fact, stalk cells usually divide at a relatively low rate. They are required wherever there is a recurring need to replace differentiated cells that cannot themselves divide, and this includes a great variety of tissues. Thus stalk cells are of many types, specialized for the genesis of unlike classes of terminally differentiated cells—epidermal stem cells for epidermis, intestinal stem cells for abdominal epithelium, hemopoietic stem cells for blood, and so on. Each stem-cell system nevertheless raises like cardinal questions. What factors determine whether the stem cell divides or stays quiescent? What decides whether a given daughter cell differentiates or remains a stem cell? And where the stalk jail cell can give ascension to more than one kind of differentiated prison cell—as is very often the case—what determines which differentiation pathway is followed?
The 2 Daughters of a Stalk Cell Do Not Always Accept to Go Different
At steady state, to maintain a stable stem-cell population, precisely 50% of the daughters of stem cells in each prison cell generation must remain every bit stem cells. In principle, this could exist achieved in two means—through environmental asymmetry or through divisional asymmetry (Figure 22-5). In the one strategy, the division of a stalk cell could generate two initially similar daughters whose fates would exist governed by their subsequent environment; 50% of the population of daughters would remain as stem cells, but the two daughters of an individual stem jail cell in the population might ofttimes have the same fate. At the opposite extreme, the stem cell division could be always strictly asymmetric, producing i daughter that inherits the stem-jail cell character and another that inherits factors that forcefulness it to embark on differentiation. In the latter case, the existing stalk cells could never increase their numbers, and any loss of stalk cells would be irreparable.

Effigy 22-5
Two means for a stem cell to produce daughters with different fates. In the strategy based on environmental asymmetry, the daughters of the stem cell are initially similar and are directed into unlike pathways according to the environmental influences (more...)
In fact, if a patch of epidermis is destroyed, the damage is repaired by surrounding epidermal cells that migrate in and proliferate to cover the denuded area. In this process, a new cocky-renewing patch of epidermis is established, implying that additional stem cells accept been generated to make upwards for the loss. These must have been produced by symmetric divisions in which one stalk cell gives rise to two. In this manner, the stem cell population adjusts its numbers to fit the available niche.
Observations such as these propose that the maintenance of stem jail cell graphic symbol in the epidermis might be controlled by contact with the basal lamina, with a loss of contact triggering the kickoff of terminal differentiation, and maintenance of contact tending to preserve stem cell potential. This idea contains a grain of truth, simply it is not the whole truth, equally nosotros now explicate.
The Basal Layer Contains Both Stalk Cells and Transit Amplifying Cells
Basal keratinocytes can be dissociated from intact epidermis and tin proliferate in a civilisation dish, giving rise to new basal cells and to terminally differentiated cells. Fifty-fifty within a population of cultured basal keratinocytes that all seem undifferentiated, at that place is cracking variation in the ability to proliferate. When cells are taken singly and tested for their ability to found new colonies, some seem unable to divide at all, others go through just a few segmentation cycles so halt, and still others divide enough times to form big colonies. This proliferative potential directly correlates with the expression of the β1 subunit of integrin (run across Effigy nineteen-64). Clusters of cells with high levels of this molecule can be found in the basal layer of the intact human epidermis too, and they are idea to be the stem cells (Figure 22-six).

Effigy 22-6
The distribution of stem cells in human being epidermis, and the blueprint of epidermal jail cell production. The diagram is based on specimens in which the location of the stem cells was identified by staining for β1-integrin, and that of the differentiating (more...)
Basal cells expressing β1 integrin at a lower level tin can also divide—indeed, they divide more frequently—merely simply for a limited number of division cycles, later on which they go out the basal layer and differentiate. These latter cells are chosen transit amplifying cells—"transit", because they are in transit from a stem-cell character to a differentiated character; "amplifying", considering the segmentation cycles they go through have the upshot of amplifying the number of differentiated progeny that event from a unmarried stem-cell division (Effigy 22-7). Mingled with the population of transit amplifying cells are some cells, still connected with the basal lamina past a thin stem, that have already stopped dividing and begun to differentiate, every bit indicated by the types of keratin molecules they express. Contact with the basal lamina, therefore, cannot exist the only factor controlling the developmental fate of an epidermal basal cell.
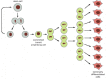
Figure 22-7
Transit amplifying cells. Stem cells in many tissues divide only rarely, but requite rising to transit amplifying cells—daughters committed to differentiation that go through a limited serial of more rapid divisions earlier completing the procedure. In (more...)
This is not to say that contact with the basal lamina or a similar substratum does non affair. If cultured basal keratinocytes are held in suspension, instead of being allowed to settle and adhere to the bottom of the civilization dish, they all terminate dividing and differentiate. To remain as an epidermal stem cell, it is apparently necessary for information technology to be attached to the basal lamina or other extracellular matrix, even though it is not sufficient. This requirement helps ensure that the size of the stalk cell population does not increase without limit. If crowded out of their regular niche on the basal lamina, the cells lose their stem jail cell character. When this rule is broken, every bit in some cancers, the result can be an e'er-growing tumor.
Epidermal Renewal Is Governed by Many Interacting Signals
Cell turnover in the epidermis seems at outset glance a simple matter, but the simplicity, equally we have just seen, is deceptive. There are many points in the process that have to exist controlled according to circumstances: the rate of stem-jail cell division; the probability that a stem-jail cell girl will remain a stem cell; the number of cell divisions of the transit amplifying cells; the timing of exit from the basal layer, and the time that the cell and then takes to complete its differentiation plan and exist sloughed from the surface. Regulation of these steps must enable the epidermis to respond to crude usage past becoming thick and callused, and to repair itself when wounded. In specialized regions of epidermis, such equally those that form pilus follicles, with their own specialized subtypes of stem cells, yet more controls are needed to organize the local pattern.
Each control bespeak is of import in its own mode, and a whole panoply of molecular signals regulate them, so equally to keep the body surface always properly covered. Almost of the prison cell communication mechanisms described in Chapter 15 are implicated, either in signaling between cells within the epidermis or in signaling between epidermis and dermis. The EGF, FGF, Wnt, Hedgehog, Notch, BMP/TGFβ, and integrin signaling pathways are all involved (and we shall encounter that the same is truthful of almost other tissues). Mutations in components of the Hedgehog or Wnt pathways, for example, can lead to the development of epidermal cancers. Components of the Hedgehog, Notch, BMP, and Wnt pathways when misexpressed interfere with the formation of hairs, blocking their evolution or causing them to develop out of place.
Activation of the Wnt pathway seems to favor maintenance of stem-cell graphic symbol, inhibiting the switch from stem cell to transit amplifying cell, whereas Notch signaling in the epidermis seems to have a opposite event, inhibiting neighbors of stem cells from remaining equally stem cells. TGFβ has a key role in signaling to the dermis during the repair of peel wounds, promoting the germination of collagen-rich scar tissue. And integrins in the epidermis are not merely markers of cell graphic symbol, but also regulators of cell fate. Thus, when transgenic mice are engineered to maintain in upper epidermal layers the expression of integrins normally bars to the basal layer, they develop a condition resembling the common human being pare disorder psoriasis: the charge per unit of basal cell proliferation is profoundly increased, the epidermis thickens, and cells are shed from the surface of the peel within as niggling as a week after emerging from the basal layer, earlier they have had time to keratinize fully. The precise individual functions of all the various signaling mechanisms in the epidermis are simply beginning to be disentangled.
The Mammary Gland Undergoes Cycles of Evolution and Regression
In specialized regions of the body surface, other types of cells also the keratinized cells described above develop from the embryonic epidermis. In detail, secretions such as sweat, tears, saliva, and milk are produced by cells segregated in deep-lying glands that originate equally ingrowths of the epidermis. These epithelial structures have functions and patterns of renewal quite different from those of keratinizing regions.
The mammary glands are the largest and most remarkable of these secretory organs. They are the defining characteristic of mammals and an important concern in many ways: not only for nourishment of babies and allure of the opposite sex, simply also as the basis for a large manufacture—the dairy manufacture—and as the site of some of the commonest forms of cancer. Mammary tissue illustrates about dramatically that developmental processes proceed in the adult body; and information technology shows how cell death past apoptosis can let development to get into reverse.
Milk product must be switched on when a infant is built-in and switched off when the infant is weaned. A "resting" developed mammary gland consists of branching systems of ducts embedded in fatty connective tissue. The ducts are lined past an epithelium that includes a subpopulation of mammary stem cells (though the precise location of the stem cells is nonetheless debated). Equally a offset pace toward milk production, the hormones that circulate during pregnancy crusade the duct cells to proliferate, increasing their numbers tenfold or twentyfold. The terminal portions of the ducts grow and co-operative, forming trivial dilated outpocketings, or alveoli, containing secretory cells (Effigy 22-8). Milk secretion begins only when these cells are stimulated by the dissimilar combination of hormones that circulate in the mother after the nascency of the baby. A further tier of hormonal control governs the bodily ejection of milk from the chest: the stimulus of suckling causes cells of the hypothalamus (in the brain) to release the hormone oxytocin, which travels via the bloodstream to act on myoepithelial cells. These musclelike cells originate from the same epithelial precursor population as the secretory cells of the breast, and they have long spidery processes that embrace the alveoli. In response to oxytocin they contract, thereby squirting milk out of the alveoli into the ducts.

Effigy 22-8
The mammary gland. (A) The growth of alveoli from the ducts of the mammary gland during pregnancy and lactation. Only a small part of the gland is shown. The "resting" gland contains a minor corporeality of inactive glandular tissue embedded (more than...)
Somewhen, when the baby is weaned and suckling stops, the secretory cells die past apoptosis, and virtually of the alveoli disappear. Macrophages apace clear away the expressionless cells, and the gland reverts to its resting state. This ending of lactation is abrupt and, unlike the events that lead up to information technology, seems to be induced by the aggregating of milk, rather than by a hormonal mechanism. If one subset of mammary ducts is obstructed so that no milk can be discharged, the secretory cells that supply it commit mass suicide past apoptosis, while other regions of the gland survive and continue to office. The apoptosis is triggered past a combination of factors including TGFβ3, which accumulates where milk secretion is blocked (Figure 22-9).

Figure 22-ix
Death of milk-secreting cells when suckling stops. (A) A section of part of a lactating mammary gland of a mouse that had been suckling her newborn babies in the normal way. (B) A corresponding section from a mouse that had not suckled for nine hours. The (more...)
Prison cell partition in the growing mammary gland is regulated not only by hormones but also by local signals passing betwixt cells inside the epithelium and betwixt the epithelial cells and the connective tissue, or stroma, in which the epithelial cells are embedded. All the signals listed earlier equally important in controlling cell turnover in the epidermis are besides implicated in decision-making events in the mammary gland. Again, signals delivered via integrins play a crucial function: deprived of the basal lamina adhesions that activate integrin signaling, the epithelial cells fail to reply unremarkably to hormonal signals. Faults in these interacting control systems underlie some of the commonest forms of cancer, and nosotros need to understand them better.
Summary
Pare consists of a tough connective tissue, the dermis, overlaid by a multilayered waterproof epithelium, the epidermis. The epidermis is continually renewed from stem cells, with a turnover fourth dimension, in humans, of the order of a month. Stalk cells, by definition, are not terminally differentiated and take the power to divide throughout the lifetime of the organism, yielding some progeny that differentiate and others that remain stem cells. The epidermal stem cells lie in the basal layer, attached to the basal lamina. Progeny that get committed to differentiation get through several rapid divisions in the basal layer, and then stop dividing and move out toward the surface of the skin. They progressively differentiate, switching from expression of one set up of keratins to expression of another until, eventually, their nuclei degenerate, producing an outer layer of dead keratinized cells that are continually shed from the surface.
The fate of the daughters of a stem cell is controlled in part by interactions with the basal lamina and in office by a multifariousness of signals from neighboring cells. These controls allow 2 stem cells to exist generated from one during repair processes, and they regulate the rate of basal cell proliferation according to need. Glands connected to the epidermis, such as the mammary glands, take their own stem cells and their own distinct patterns of cell turnover, governed by other conditions. In the breast, for case, circulating hormones stimulate the cells to proliferate, differentiate, and make milk; the abeyance of suckling triggers the milk-secreting cells to die by apoptosis, as a outcome of a build-up of TGFβ3 where milk fails to be drained away.
Source: https://www.ncbi.nlm.nih.gov/books/NBK26865/
Posted by: kylecionarsellar.blogspot.com



0 Response to "How Do Skin Cells Divide"
Post a Comment