Are Chemicals Absorbed Through Skin
Peel assimilation is a route by which substances tin enter the torso through the skin. Along with inhalation, ingestion and injection, dermal absorption is a route of exposure for toxic substances and route of administration for medication. Absorption of substances through the skin depends on a number of factors, the nigh important of which are concentration, elapsing of contact, solubility of medication, and physical condition of the pare and role of the trunk exposed.
Pare (percutaneous, dermal) absorption is the send of chemicals from the outer surface of the skin both into the skin and into circulation. Skin absorption relates to the degree of exposure to and possible effect of a substance which may enter the body through the peel. Human pare comes into contact with many agents intentionally and unintentionally. Skin absorption can occur from occupational, environmental, or consumer skin exposure to chemicals, cosmetics, or pharmaceutical products. Some chemicals can be absorbed in plenty quantity to crusade detrimental systemic effects. Pare disease (dermatitis) is considered one of the nearly common occupational diseases.[one] In order to assess if a chemical can be a risk of either causing dermatitis or other more systemic effects and how that adventure may be reduced i must know the extent to which it is absorbed, thus dermal exposure is a key aspect of human wellness risk assessment.
Factors influencing absorption [edit]
Forth with inhalation, ingestion and injection, dermal absorption is a route of exposure for bioactive substances including medications.[two] Absorption of substances through the skin depends on a number of factors:
- Concentration
- Molecular weight of the molecule[3]
- Duration of contact
- Solubility of medication
- Physical condition of the pare
- Office of the torso exposed including the amount of hair on the skin
In general, the rate of assimilation of chemicals through peel follows the post-obit scheme from fastest to slowest: Scrotal > Forehead > Armpit ≥ Scalp > Back = Abdomen > Palm = under surface of the foot.[four]
Structures influencing assimilation [edit]
To be captivated through the skin, a chemical must pass through the epidermis, glands, or hair follicles. Sweat glands and hair follicles make up about 0.1 to 1.0 pct of the total skin surface.[2] Though small amounts of chemicals may enter the trunk rapidly through the glands or pilus follicles, they are primarily captivated through the epidermis. Chemicals must pass through the seven cell layers of epidermis before inbound the dermis where they can enter the blood stream or lymph and broadcast to other areas of the torso. Toxins and toxicants tin move through the layers by passive diffusion. The stratum corneum is the outermost layer of the epidermis and the rate-limiting barrier in assimilation of an agent.[four] Thus, how quickly something passes through this thicker outer layer determines the overall absorption. The stratum corneum is primarily composed of lipophilic cholesterol, cholesterol esters and ceramides. Thus lipid-soluble chemicals brand it through the layer and into the apportionment faster, all the same nearly all molecules penetrate it to some minimal degree.[5] [6] Absorption of chemicals in municipal h2o and dental products such every bit VOC (Volatile Organic Compounds), TTHM (Total Trihalomethanes), fluoride and disinfectants is a major exposure to environmental health hazards.[7] [viii] [9]
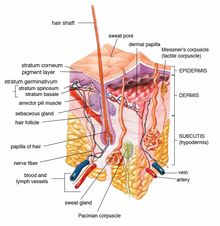
Diagram of peel structures.
Conditions affecting peel absorption [edit]
Agents that injure the stratum corneum, such as strong acids, are absorbed faster than chemicals that do not.[x] Skin damage due to burns, abrasions, wounds and pare diseases also increase absorption. Thus populations with skin damage may be more susceptible to adverse effects of agents that are absorbed through the peel. Sure solvents like dimethyl sulfoxide (DMSO) act as carriers and are oft used to send medication through the skin. DMSO increases the permeability of the stratum corneum.[11] [12] Surfactants like sodium lauryl-sulfate increase the skin penetration of h2o-soluble substances, possibly by increasing the skin permeability of water.[11]
Medical use of skin assimilation [edit]
Dermal application of a medication or chemical allows treatment to be localized, unlike ingestion or injection. Some medications seem to be more constructive (or are more efficient) using the dermal route of administration. Some ingested drugs are heavily metabolized by the liver and may exist inactivated, only using a dermal awarding bypasses this metabolic footstep allowing more parent compounds to enter the peripheral circulation. If a drug is absorbed well through the pare it may be used as a means of systemic medication. Dermal dosage forms include: liniments, braces, lotions, ointments, creams, dusting powders, aerosols, and transdermal patches.[thirteen] Specially designed patches are currently used to evangelize fentanyl, nicotine and other compounds. Slower skin absorption versus oral or injectable may allow patches to provide medication for i to 7 days.[fourteen] For example nitroglycerin given transdermally may provide hours of protection against angina whereas the elapsing of outcome sublingually may only exist minutes.[15]
Measurement of skin absorption [edit]
The corporeality of chemical that is absorbed through the peel can be measured straight or indirectly.[sixteen] Studies accept shown there are species with differences in the absorption of different chemicals. Measurements in rats, rabbits or pigs may or may non reflect human absorption.[ten] Finding the rate at which agents penetrate the pare is of import for assessing the risk from exposures.
Directly measurement [edit]
In vivo [edit]
The transit of chemicals into the skin can be straight measured using not-invasive optical techniques with molecular specificity, such as Confocal Raman Spectroscopy. This technique is able to identify unique spectra of molecules and compare to background skin spectra whilst limiting measurement regions using confocal gating, achieving depth-resolved concentration measurement. A single measurement sequence can thereby establish a snapshot profile of chemical concentration against depth inside the skin. By repeating the measurement at multiple timepoints, a dynamic concentration-at-depth contour is adamant. Since modern Raman Spectrometers showroom extremely high SNR, in-vivo absorption testing in human skin is possible on a scale of a few minutes or hours.
A chemical may likewise be directly applied to the skin followed by blood and urine measurements, at set time points after the awarding, to assess the corporeality of chemical that entered the trunk. The concentration in the blood or urine at particular time points tin can exist graphed to show an expanse under the curve and the extent and duration of absorption and distribution to provide a measure out of systemic absorption. This can be done in animals or humans with a dry chemic powder or a chemical in solution.[17] Rats are normally used for these experiments. An area of skin is shaved before the chemic is applied. Ofttimes the expanse of chemical awarding is covered to prevent ingestion or rubbing off of the test textile. Samples of blood and urine are taken at specific time intervals following awarding (0.5, 1, 2, 4, 10, and 24 hours) and in some protocols at the chosen end fourth dimension the beast maybe necropsied. Tissue samples may also be evaluated for the presence of the test chemical.[xviii] In some test protocols many animals may be tested and necropsies may occur at gear up intervals after exposure. Biomonitoring, such as taking urine samples at intervals, from workers exposed to chemicals may provide some data but it is difficult to distinguish dermal from inhalation exposure using this method.
Ex vivo [edit]
The permeability properties of the stratum corneum are, for the near function, unchanged after its removal from the body.[18] Skin that has been removed carefully from animals may besides be used to see the extent of local penetration by putting it in a chamber and applying the chemical on 1 side and and so measuring the amount of chemical that gets into a fluid on the other side.[14] One example of this ex vivo technique is the isolated perfused porcine flap.[4] This method was start described in 1986 as a humane alternative to in vivo fauna testing.[19]
In vitro [edit]
Techniques such as static diffusion cells (Franz cells) and flow-through improvidence cells (Bronaugh cells) take also been used.[4] The Franz Prison cell apparatus consists of two chambers separated by a membrane of beast or human skin. Homo skin is preferred but due to ethical and other considerations is not ever bachelor. Homo pare oft may come from autopsies or plastic surgeries.[20] The test product is applied to the membrane via the elevation bedroom. The bottom sleeping room contains fluid from which samples are taken at regular intervals for analysis to decide the corporeality of agile cells that has permeated the membrane at set time points.
Bronaugh cells are similar to Franz cells just utilise a flow-through system below the membrane layer and samples of the liquid below are taken continuously rather than at prepare time points.[21] Bronaugh cells have been replaced with inline cells past some manufacturers.
Indirect measurement [edit]
Information technology is sometimes impossible for humane reasons to utilize a drug to the skin and measure its assimilation. Sarin, a nerve gas, can be absorbed through intact pare and be lethal at low concentrations. Thus if one needs to assess the risk of Sarin exposure one must take skin absorption and other routes into account but one cannot ethically test Sarin on man subjects; thus ways of modeling the run a risk from pare exposure of the agent have been found.
Models are used in some instances to predict the amount of exposure or absorption and to assess public health hazards. In order to appraise the take a chance of a chemical causing a health issue i must assess the chemical and the exposure. Exposure modeling depends on several factors and assumptions.
- The surface surface area of peel exposed. The surface area of an developed is about twenty,900 square centimeters (iii,240 sq in) and the surface area of a child of 6 years is almost 9,000 foursquare centimeters (1,400 sq in). These figures and figures for other trunk parts or portions can be found in the EPA (Environmental Protection Agency) Exposures Handbook 1996[22] or estimated using other databases.[23]
- The duration of exposure (in hours, minutes, etc.).
- The concentration of the chemical.
- The permeability coefficient of the chemical (how easy it is for the chemical to get through the pare). This may be estimated using an octanol-h2o sectionalization coefficient (a measurement of the uptake from aqueous solution into powdered stratum corneum).[24]
- The weight of the person. Standard weight of an adult 71.eight kg, a 6-year-old child 22 kg and female person of changeable age threescore kg are generally used.[22]
- The nature of the exposure, east.k. a cream applied to the whole body, to just a pocket-size area, or a bath in a dilute solution.
Skin contact with dry chemic [edit]
- To calculate the dose of chemical a person is exposed to one must multiply the surface area of the skin exposed by the concentration of the chemical in the substance that comes into contact with the pare. Then multiply by the time in contact, by the permeability coefficients, and whatever unit conversion factors needed, and so divide by the weight of the person.
- A unproblematic mathematical formula to estimate the dose for a unmarried exposure is:
- concentration of chemical × surface area exposed × permeability coefficient / body weight.
- Models for this can be found in the EPA Standard Operating Procedures for Residential Exposure Assessment.[25] These models establish guidelines for estimating pesticide exposure so that one tin gauge the risk and have appropriate actions if the risk is judged to be too bang-up given the exposure.
Skin contact with chemical in solution (h2o, etc.) [edit]
- This can be modeled similarly to the dry chemical merely one has to take into business relationship the corporeality of solution the skin comes into contact with. 3 scenarios for exposure to chemicals in a solution take been proposed and modeled.
- a. A person could be exposed partially to a solution for a period of fourth dimension. For case if one stood in contaminated flood water for a period of time, or ane worked in a state of affairs where the hands and lower arms were immersed in a solution for a flow of time. This blazon of scenario depends on the peel surface area exposed and the duration of the exposure as well as the concentration of the chemic in the solution. One may have to adjust the absorption coefficients for the different expanse of the body as the feet are more calloused on the bottom and will allow less chemical through than the lower leg. The rate of assimilation of chemicals follows the following full general scheme from fastest to slowest: Scrotal > Forehead > Armpit ≥ Scalp > Back = Abdomen > Palm = under the surface of the human foot.[4] The dermal absorption of a dilute solution by partial leg or arm exposure has been modeled by Scharf.[17] The EPA also has guidance on calculating the dermally absorbed doses of chemicals from contaminated water.[26]
- Mathematical formula:
- Dermally absorbed dose rate = concentration in water × surface expanse exposed × exposure time × permeability coefficient × conversion factors .
- b. The 2nd scenario is total body immersion, such as pond in a pool or lake. Exposure in pond pools is only partially dermal and a SWIMODEL has been proposed.[27] This model takes into account not only the pare exposure but also considers ocular, ingestion, inhalation, and mucous membrane exposure that may occur due to being totally immersed. A second model dealing primarily with skin absorption was created by Scharf to assess the risk of overspray of pesticide from aerial spraying on swimming pools.[17] These models employ whole body area rather than the surface area of specific parts for the mathematical input.
- c. The third scenario is splash, or droplet exposure. This model takes into account that not all water carrying a chemical that comes into contact with skin stays on the skin long plenty to permit assimilation. Only that portion of a chemic in the solution that stays in contact with the skin is available for absorption. This may be modeled using water adherence factors as postulated past Gujral 2011.[28]
Pare contact with gas or aerosol [edit]
- This is a minor contributor and has been ignored in virtually risk assessments of chemicals as a road of exposure for gaseous or aerosolized toxicants. More research is needed in this area.[29]
Controlling pare assimilation [edit]
If skin exposure and absorption are accounted to indicate a risk, various methods to reduce assimilation tin be undertaken.
- Labels of chemicals can be adjusted to require the employ of gloves or protective wearable.
- Warnings to wash immediately if the chemical comes into contact with skin can exist made.
- Close pools or lakes to swimmers.
- Limit the exposure fourth dimension to chemicals, i.e. workers can just work with certain chemicals for a sure length of fourth dimension per 24-hour interval.
See besides [edit]
- Assimilation (chemistry)
- Absorption (pharmacokinetics)
- Dermal patch
- Epidermis (skin)
- Exposure cess
- Exposure to toxins
- Topical medication
References [edit]
- ^ Workplace Safety & Wellness Topics: Skin Exposures & Effects. CDC. Retrieved Apr 17, 2014.
- ^ a b Eaton, DL and Klaassen Curtis D. Principles of Toxicology. in Cassarett & Doull's Toxicology, The Basic Science of Poisons. 5th edition. 1996. McGraw-Hill.
- ^ Bos, JD; Meinardi, MM (2000). "The 500 Dalton dominion for the skin penetration of chemic compounds and drugs". Exp. Dermatol. nine (3): 165–9. doi:10.1034/j.1600-0625.2000.009003165.ten. PMID 10839713.
- ^ a b c d e Baynes, RE and Hodgson E. Absorption and Distribution of Toxicants. in Chapter six of A Textbook of modern toxicology. third edition. 2004, John Wiley & Sons, Inc.
- ^ Morganti, P., Ruocco, E., Wolf, R., & Ruocco, V. (2001). "Percutaneous absorption and delivery systems." Clin Dermatol. nineteen: 489-501.
- ^ Hood, Ernie (2005). "Tap H2o and Trihalomethanes: Menses of Concerns Continues". Environmental Health Perspectives. 113 (7): A474. doi:10.1289/ehp.113-a474. PMC1257669.
- ^ Jaccobson, APM; Stephen, KW; Strang, R (1992). "Fluoride Uptake and Clearance from the Buccal Mucosa following Mouthrinsing". Caries Res. 26 (1): 56–58. doi:ten.1159/000261428. PMID 1568238.
- ^ Gabler, WL (1968). "Absorption of fluoride through the oral mucosa of rats". Curvation Oral Biol. 13 (six): 619–623. doi:x.1016/0003-9969(68)90140-4. PMID 5244286.
- ^ Brown, H.S.; Bishop, D.R.; Rowan, C.A. (1984). "The role of skin absorption as a road of exposure for VOCs in drinking water". Am. J. Public Health. 74 (v): 479–84. doi:x.2105/AJPH.74.5.479. PMC1651599. PMID 6711723.
- ^ a b Rozman, KK and Klaassen CD. Absorption, Distribution and Excretion of Toxicants. in Cassarett & Doull'due south Toxicology, The Basic Scientific discipline of Poisons. 5th edition. 1996. McGraw-Loma
- ^ a b Baggot JD. Disposition and Fate of Drugs in the Body. Affiliate five in Veterinarian Pharmacology and Therapeutics, 6th edition, 1988 Iowa Country Press, Ames.
- ^ Booth NH, Topical Agents. Chap 44 in Veterinary Pharmacology and Therapeutics, 6th edition, 1988 Iowa State Press, Ames.
- ^ Davis, LE. Drug presentation and prescribing. Chap 3 in Veterinarian Pharmacology and Therapeutics, 6th edition, 1988 Iowa State Press, Ames.
- ^ a b Rice, RH and Cohen DE. Toxic Responses of the Skin. in Cassarett & Doull's Toxicology. The Basic Science of Poisons. 5th Edition. 1996. McGraw-Hill
- ^ Shargel, L and Yu, A. Chapter 11. Modified-release drug products and drug delivery systems. in Practical Biopharmaceuts and Pharmacokinetics. 3rd edition. 1993 Appleton & Lange.
- ^ Musazzi, Umberto K.; Matera, Carlo; Dallanoce, Clelia; Vacondio, Federica; De Amici, Marco; Vistoli, Giulio; Cilurzo, Francesco; Minghetti, Paola (2015). "On the selection of an opioid for local skin analgesia: Structure-peel permeability relationships". International Periodical of Pharmaceutics. 489 (1–2): 177–185. doi:10.1016/j.ijpharm.2015.04.071. ISSN 0378-5173. PMID 25934430.
- ^ a b c Scharf, JE; et al. (2008). "Dermal absorption of a dilute aqueous solution of malathion". J. Emerg. Trauma Shock. i (2): lxx–73. doi:ten.4103/0974-2700.43182. PMC2700616. PMID 19561983.
- ^ a b Earth Health System, Environmental Health Criteria 235, Dermal Absorption, 2006.
- ^ Riviere JE et al. The isolated perfused porcine pare flap (IPPSF). I. A novel in vitro model for percutaneous absorption and cutaneous toxicology studies. Fundam Appl Toxicol. 1986 Oct;7(iii):444-53.
- ^ Dressler We (1999) Hair dye absorption. In: Bronaugh RL & Maibach HI eds. Percutaneous absorption: drugs–cosmetics–mechanisms–methodology, tertiary ed. New York, Marcel Dekker, pp 685–716 (Drugs and the Pharmaceutical Sciences Vol. 97).
- ^ Bronaugh, R.L.; Stewart, R.F. (1985). "Methods for percutaneous absorption studies. IV. The flowthrough diffusion cell". J. Pharm. Sci. 74 (one): 64–67. doi:ten.1002/jps.2600740117. PMID 3981421.
- ^ a b EPA Exposure Handbook 1996
- ^ Yu, CY et al. Human being torso surface area database and estimation formula. Burns. 2010 Aug;36(5):616-29..
- ^ Wester; et al. (1987). "In vivo and vitro binding to powdered human stratum corneum every bit methods to evaluate peel absorption of environmental chemical contaminiants from ground and surface h2o". J Toxicol Environ Health. 21 (3): 367–374. doi:10.1080/15287398709531025. PMID 3108517.
- ^ EPA 2012 Standard Functioning Procedures (SOPs) for Residential Exposure Assessment
- ^ US Enivironmental Protection Bureau. Risk Assessment Guidance for Superfund. Book I: Human Health Evaluation Manual (Part E, Supplemental Guidance for Dermal Risk Assessment)-Final. Washington, DC: United states EPA, Function of Superfund Remediation and Technology Innovation, EPA/540/R/99/005, OSWER 9285.seven-02EP, July 2004.
- ^ Dang, 1996 EPA SOP Estimating post application dermally absorbed dose from chemicals in Swimming pools
- ^ Gujral, J. S.; Proctor, D. M.; Su, S. H.; Fedoruk, J. M. (2011). "Water Adherence Factors for Man Skin". Run a risk Analysis. 31 (8): 1271–1280. doi:10.1111/j.1539-6924.2011.01601.x. PMID 21453376.
- ^ Rauma, M.; et al. (Feb 2013). "Predicting the absorption of chemical vapours". Adv Drug Deliv Rev. 65 (2): 306–fourteen. doi:10.1016/j.addr.2012.03.012. PMID 22465561.
External links [edit]
- Skin Exposures & Effects, Centers for Disease Control and Prevention
- EDETOX database
- WHO, Environmental Wellness Criteria 235, Dermal Absorption
- EPA 2012 Standard Operation Procedures (SOPs) for Residential Exposure Assessment
- Description of Franz cells
- [1]
Source: https://en.wikipedia.org/wiki/Absorption_(skin)
Posted by: kylecionarsellar.blogspot.com


0 Response to "Are Chemicals Absorbed Through Skin"
Post a Comment